June 17, 2016 – In Alzheimer’s Disease (AD), intracellular neurofibrillary tangles (tau) pathology may prove to be an instrumental target for disease-modifying strategies in AD, and extracellular beta-amyloid plaques (Aβ) aggregation plays an indirect role in causing neurodegeneration, according to findings in a study1 presented at SNMMI’s 63rd Annual Meeting, June 11–15, 2016, San Diego, Calif.
In Alzheimer’s Disease (AD), Aβ and tau represent the primary pathological hallmarks, however, the degree to which the accumulation of these proteins contributes to neurodegenerative disease remains unclear. Despite advances in molecular imaging, the researchers write in their abstract that studies have not established a direct relationship between Aβ-deposition (as measured with [11C]PiB PET) and regional neurodegeneration (as measured with [18F]FDG-PET). With the advent of a novel PET tracer for tau imaging ([18F]AV-1451 aka T807) that shows high affinity to intracellular tangle pathology, it may now be possible to establish the missing link between protein aggregation pathology and neurodegeneration.
The researchers of this study assessed the relationship of tau and Aβ pathology to regional and global measures of hypometabolism in AD patients using multimodal PET. To assessed glucose hypometabolism using [18F]FDG PET, measuring tau deposition using [18F]AV-1451 PET in conjunction with [18F]FDG PET and [11C]PiB PET, and Aβ ([11C]PiB PET) in ten AD patients. They found a strong linear relationship between tau deposition and hypometabolism (r=.71, p<.001), whereas measures of Aβ were not related to hypometabolism (r=-.17, ns). Within regions, association of tau deposition and hypometabolism was most pronounced in the parietal, temporal and occipital lobe.
They concluded that the findings provide evidence for the hypothesis that tau pathology may represent a direct instigator of neuronal injury and, thus, of regional neurodegeneration in AD. In contrast, effects of Aβ deposition on neuronal function (if present) were observed only in dependence of tau pathology. This implicates a more indirect role of Aβ aggregation in causing neurodegeneration. This study emphasizes that tau pathology may prove to be an instrumental target for disease-modifying strategies in AD.
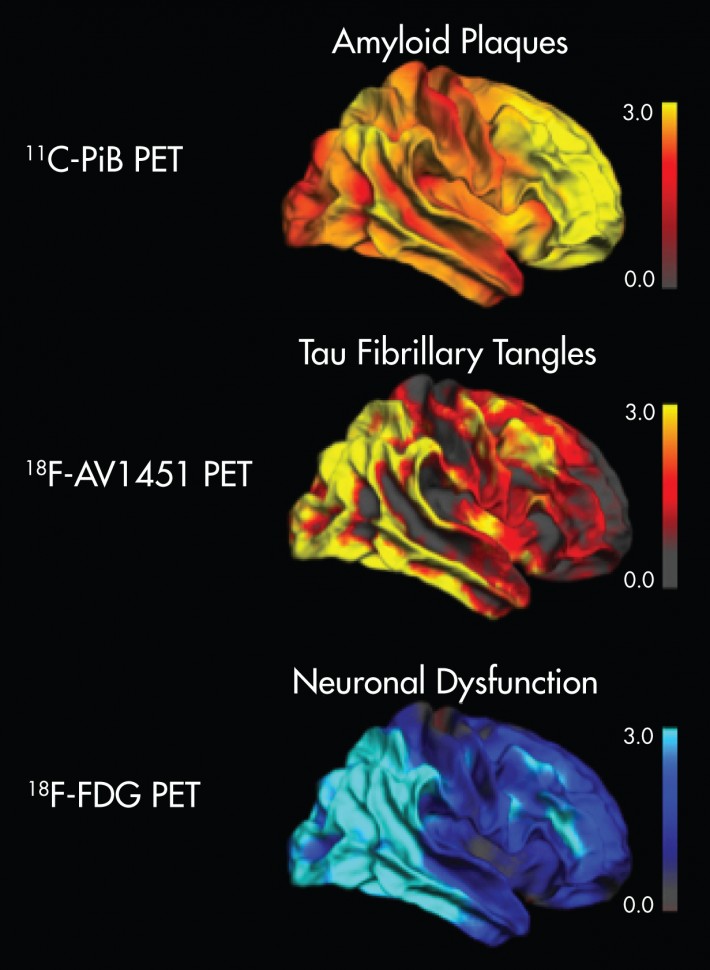
Figure: Image of the Year at SNMMI’s 63rd Annual Meeting, June 11–15, 2016, San Diego, Calif.2
References:
1. Bischof G, Hammes J, Eimeren V, Drzezga A. Differential contributions of Amyloid and Tau burden to Neurodegeneration in Alzheimer’s Disease: A multimodal in vivo PET study. J Nucl Med. May 1, 2016 vol. 57 no. supplement 2 124. http://jnm.snmjournals.org/content/57/supplement_2/124.short?cited-by=yes&legid=jnumed;57/supplement_2/124
2. SNMMI Image of the Year, Scientific Paper 124: G. Bischof, J. Hammes, T. van Eimeren, and A. Drzezga, Multimodal Neuroimaging Group, Department of Nuclear Medicine, University Hospital of Cologne, Germany, and B. Neumaier, Institute of Radiochemistry and Experimental Molecular Imaging, University of Cologne, Germany. The study has been performed in close cooperation with the Departments of Neurology and Psychiatry, University of Cologne, Germany, the Research Center Jülich, Germany, and the German Center for Neurodegenerative Diseases. Presented at SNMMI’s 63rd Annual Meeting, June 11–15, 2016, San Diego, Calif.




