February 16, 2015 - The Lung-RADS criteria to the National Lung Screening Trial (NLST) may substantially reduce the false-positive result rate, however, sensitivity is also decreased, according to a recently published study.1
The objective of the study was to evaluate the effect of using Lung-RADS criteria in clinical practice. The researchers retrospectively applied the Lung-RADS criteria to the NLST using secondary analysis of a group from a randomized trial across 33 screening centers in the United States. Patients aged 55 to 74-years-old, had at least a 30-pack-year history of smoking, and were current smokers or had quit within the past 15 years, were randomly assigned to the LDCT group of the NLST.
The investigators measured the results using Lung-RADS classifications for LDCT screenings. Lung-RADS categories 1 to 2 constituted negative screening results, and categories 3 to 4 constituted positive results.
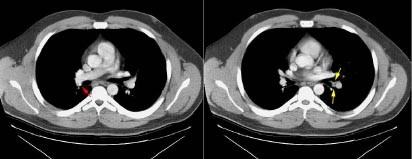
A small-calcified node is seen on the right (red arrow) in this patient with prior granulomatous disease.
The false-positive result rate for Lung-RADS after baseline declined to 5.3% compared to 12.8% at baseline; versus a drop from 26.6% at baseline for the NLST and 21.8% after baseline. The sensitivity post-baseline results for Lung-RADS went up from 78.6% to 84.9%; versus a small increase for the NLST from 93.5% at baseline to 93.8% after baseline.
The researchers concluded that Lung-RADS may substantially reduce the false-positive result rate; however, sensitivity is also decreased, and suggested that further studies should be done on Lung-RADS criteria in clinical practice.
- Pinsky PF, Gierada DS, Black W, et al. Performance of Lung-RADS in the National Lung Screening Trial: A Retrospective Assessment. Ann Intern Med. 2015 Feb 10. doi: 10.7326/M14-2086. [Epub ahead of print]




